Colloidal stability:
Over time many alcoholic beverages are likely to form a haze, show a light precipitate, or even exhibit a potential for significant turbidity which can be caused by colloidally dissolved substances. A search through the brewing literature on the topic will provide many definitions of what are considered colloidal substances:
“A colloid is a homogeneous, non-crystalline substance consisting of large molecules or ultramicroscopic finely divided particles (1 to 1000 millimicrons [= 10-9 meter or nanometers] in size) of one substance dispersed within a continuous medium in a manner that prevents them from being filtered easily or settled rapidly. Colloids include gels, sols, and emulsions; the particles do not settle and cannot be separated out by ordinary filtering or centrifuging like those in a suspension. The term also refers to the particulate matter so dispersed. Colloids may be thought of as a mixture with properties between those of a solution and a fine suspension (finely “floating” matter).”
“A colloidal gel is a colloid in a more solid form than a sol (liquid).”
“A sol is a colloidal suspension of very small solid particles in a continuous liquid medium. Sols are quite stable and show a unique light scattering pattern known as the Tyndall Effect.
“A hydrogel is a colloidal gel in which water is the dispersion medium.”
“An emulsion is a colloid consisting of a mixture of two liquids such as that of oil in water. It refers to microscopic particles of liquid dispersed in another liquid. Milk is an example whereby lipophilic (fat loving or hydrophobic = water hating) particles are dispersed in a water-based medium.”
The colloidal stability of beer refers to its propensity to form the non-biological hazes due to interactions between beer components, principally polyphenols and proteins, leading to the formation of visible precipitates. Colloid formation in beer typically presents as gelatinous or “jelly like” masses (See Figure 1).
Figure 1. Two examples of very heavy haze and appearance of particulates in beer.

As many brewers in the US move away from filtration or fail to filter their beer properly we are seeing many complaints and examples of beer such as that shown in Figure 1. The extreme use of hops in many modern US beer style examples is only exacerbating this issue we believe, through a higher input of polyphenols derived from the hops, and is one of the culprits of a decrease in colloidal stability seen in some examples of hoppier beer styles.
Beer Colloids and Haze. Beer colloidal haze is generally the result of beer protein molecules joining together with polyphenols to form molecular aggregates of a size large enough to cause visual turbidity.
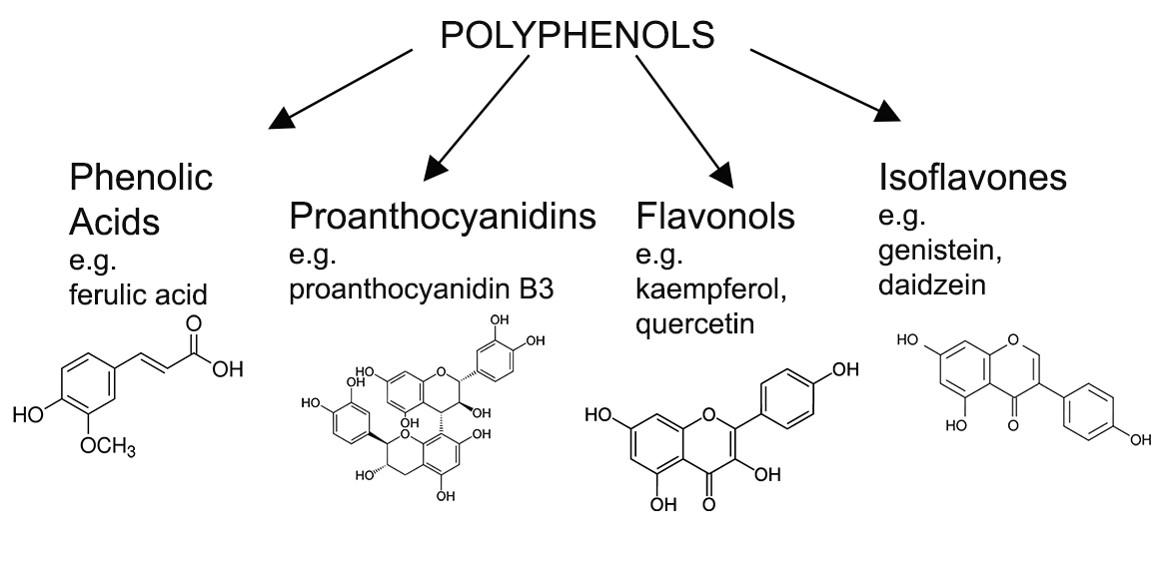
Polyphenols: These are a very diverse set of compounds which are known as polyhydroxy phenols. They commonly exist as base monomers, or as dimers, trimers and as larger, more highly complexed, polymers. The very large polymeric forms are known as tanols, tannoids, and tannins. The degree of polymerization is, however, important with respect to haze formation because dimers and trimers are much more likely to create hazes as compared with monomers. Polymers greater than trimers are not carried through the brewing process, but can form during beer storage as a result of oxidation reactions. This explains why hazes can develop with age in otherwise clear beer. Figure 2 shows a selection of polyphenol compounds with some named species.
Figure 2. An example of the complex class of biologically-derived plant molecules known as polyphenols. Polyphenols in beer are derived from grain husks during mashing and lautering operations and as extracted from hops during wort boiling. Note the monomeric and dimeric forms. Trimers and even more highly polymerized complexes are possible by addition of more polyphenols. The proanthocyanidins (also known by brewers as anthocyanogens) in particular have been associated with haze formation.
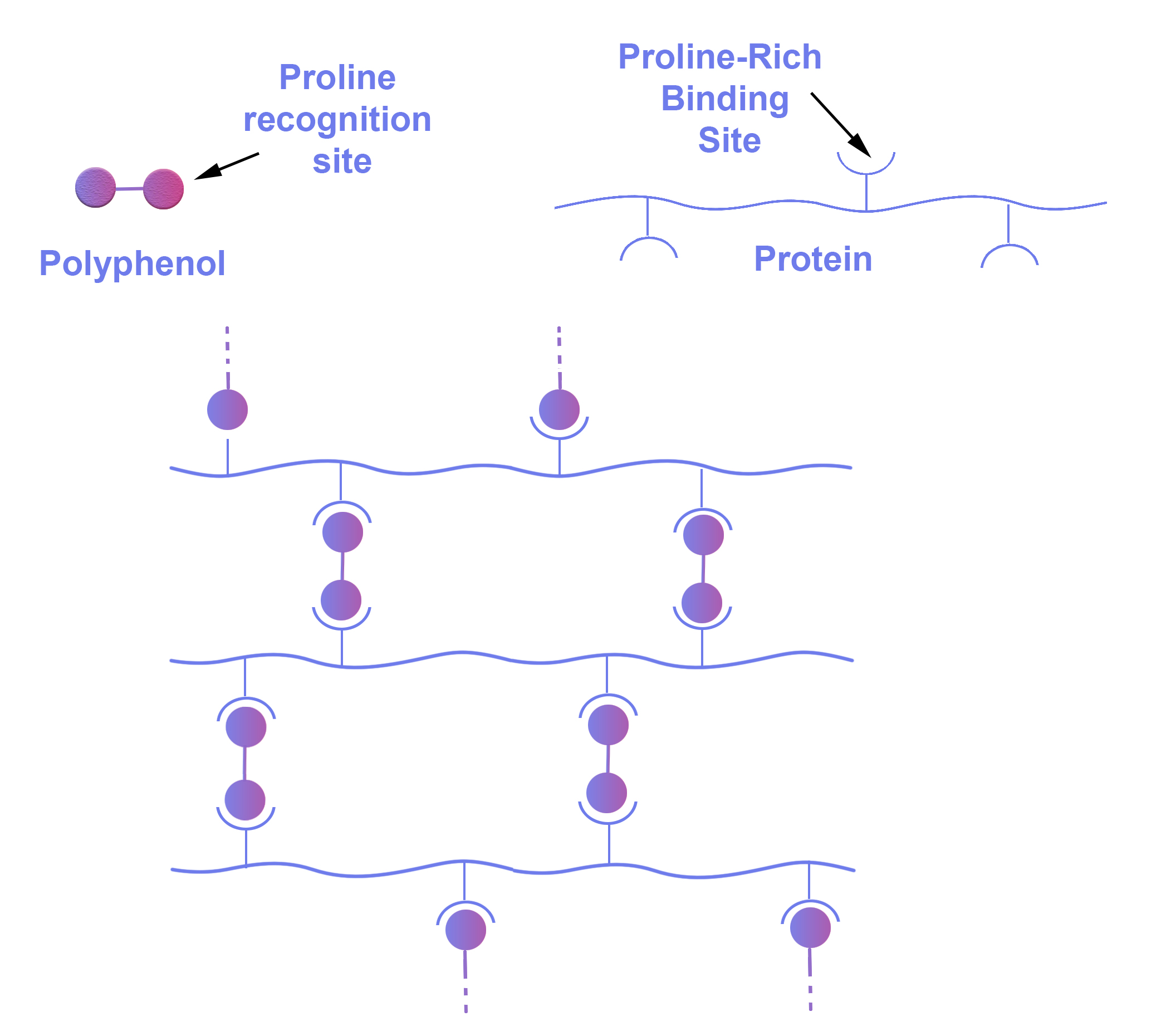
Proteins: The haze-active proteins or protein fragments (polypeptides) that are involved in protein polyphenol complexation (and derived from barley proteins) have regions rich in the amino acid proline (which is technically an imino rather than an amino acid) and it is the proline rich regions that form the binding sites for proline recognition sites on the polyphenol molecules. A schematic of the resultant protein polyphenol interaction is shown in Figure 3.
Figure 3. Protein-polyphenol complexation. A model originally defined by Prof. Karl Siebert at Cornell University in the US. Polyphenols cause the cross linking of protein/polypeptide chains.
Chill and Permanent Hazes
Colloidal hazes in beer are classified as either chill haze only occurring at low temperatures or permanent haze which is present at any temperature. Chill haze is formed at 0 °C or below but solubilizes and disappears as the beer is rewarmed back to 20 °C. The haze forms as a result of weak hydrogen bonds between small polymerized polyphenols and proteins (Figure 3).
These “weak bonds” are disrupted as the temperature rises, causing the aggregates to dissociate and the haze disappears. When beer is stored over longer time periods, further polymerization of polyphenols occurs and these much larger molecules form stronger covalent bonds with haze-sensitive proteins and the resultant hazes do not then dissolve when the beer is warmed up. Permanent hazes form in packaged beer over relatively long periods.
Controlling Colloidal Stability – At the Wort Preparation Step:
While a careful choice of raw materials and milling and mashing all have an impact on producing the precursors for colloidal instability we only consider the wort boiling and cooling processes here for assisting in colloidal stability of wort and the subsequent fermented product. Further details on these other aspects may be found in Leiper and Miedl (2009) and in a series of quality in brewing articles written by the current authors and published in the Scandinavian Brewer’s Review.
Preparing for Beer Colloidal Stability – Wort Boiling – Hot Break (aka. Trub):
Colloidal protein material is removed by wort boiling; reducing some of the coaguable nitrogen (protein) promotes colloidal stability. Boiling heat leads to coalescing and denaturing of proteins which reduces protein solubility, causing precipitation of proteins which are then more easily removed.
The production of this matter known as hot break or trub is dependent upon the physical vigor of the boil, oxygen content, reducing agents, calcium phosphate reactions and the polyphenols present. Calcium reacts with malt derived phosphates which helps to reduce the wort pH and further assists in precipitating the proteins. Kettle finings which can promote precipitation via electrostatic interactions are sometimes used to remove hot break matter.
The composition of hot break, however, not only consists of denatured precipitable protein but also insoluble salts, hop resin matter, lipids from the sweet wort and hops, polyphenols, spent hops and carbohydrates. As seen in Figure 3 protein and here in this case oxidized polyphenol complexes form which are insoluble in boiling wort and so are separated out as hot as possible. Usually today a device known as a whirlpool is used to separate out hot trub from the cleared wort.
Preparing for Beer Colloidal Stability – Wort Cooling – Cold Break (aka. Trub):
Cold break forms once wort is cooled to about 60 °C. Wort becomes cloudy with the appearance of 0.5 micrometer sized particulates – hence cold break is also known as fine or cool break. Protein- polyphenol complex formation is also involved but this time with unoxidized polyphenols, unlike in hot break, which precipitate out best at lower temperatures.
The trub here forms slowly as a finely divided precipitate and the polyphenol content is higher in cold break than in hot break. Hydrogen bonds holding the protein-polyphenol complexes together are more stable at lower temperatures. Some cold break matter is considered desirable in the wort as it ultimately rounds out beer flavor, improves beer foam, beer stability and assists the efficiency of fermentation. So today many craft brewers consider its removal as optional. If it is removed it is done so by plate and frame DE filtration, centrifugation or natural sedimentation.
Controlling Colloidal Stability – After Fermentation
From the above discussion it should hopefully be apparent that reducing the propensity for colloidal haze formation in the beer itself could involve removal of either haze-active proteins (generally hydrophilic proteins), polyphenols or both. However, a compromise is needed as proteins influence body and mouthfeel of beer and assist in foam formation (generally hydrophobic foam positive proteins).
Polyphenols are also powerful reducing agents and act as antioxidants. So, there is a balance between removal of some but not all protein and polyphenolic materials. Nevertheless, two key strategies to reduce haze formation involve physical stabilization by decreasing or removing either or both the protein levels and a bulk of the polyphenols.
Protein removal is achieved by treating the beer with silica gel – this binds protein in a similar manner to the polyphenols as seen in Figure 3 and allows the larger aggregates to be removed during filtration. Two types of silica gel adsorptive beer stabilizers are commonly used: hydrogel and xerogel (themselves colloidal substances as described in the definitions above). Silica gels are not soluble in beer and are thus also removed during the filtration step. Manufacture’s literature should be consulted for further details on the use of Silica gels for this purpose.
Removal of polyphenols on the other hand is done via the use of an agent called polyvinyl polypyrrolidone (PVPP). Insoluble in water and beer, this nylon-type polymer has a higher affinity for chemical bonding with polyphenols than the proteins present in beer. A high proportion of the polyphenols in the beer will bind to the surface of finely granulated PVPP and can then be filtered out.
It is also possible to use a combination of silica gel and PVPP with the selective removal of the protein and polyphenols in the manners described above. This can reduce the overall cost of the process as PVPP is a quite expensive commodity.
A full understanding of the conditions of use of either or both adsorbents is needed for effective and efficient removal of these haze precursors. For those wishing to delve more into the terms presented here see the series of short articles in the work by Oliver (2012).
Concluding Remarks
Brewing operations consist of a very complex series of processes which involve many chemical and biochemical reactions. Colloidal stability leads to a more stable product from a visual viewpoint by preventing the formation of hazes and unsightly precipitates in the final beer; both hot break and cold break formation and subsequent removal assists in preventing beer chill and permanent hazes!
In making readers aware of the need to remove hot break trub and sometimes cold break it is hoped that the information presented here has helped to build a better understanding of the importance of creating a colloidally stable wort and subsequent beer.
Further reading
Leiper, K.A. and Miedl, M. (2009) Colloidal stability of beer. In: Beer: A Quality Perspective. Charles W. Bamforth (Ed.). Academic Press/Elsevier.
Oliver, G. (Ed). (2012). The Oxford Companion to Beer. Oxford University Press.
BDAS, LLC. The authors have a series of articles available on many topics of interest to brewers. Available upon request. See: www.alcbevtesting.com.








