In this latest article Tim O’ Rourke, technical editor of The Brewers Journal, takes us on a journey through yeast flavour development.
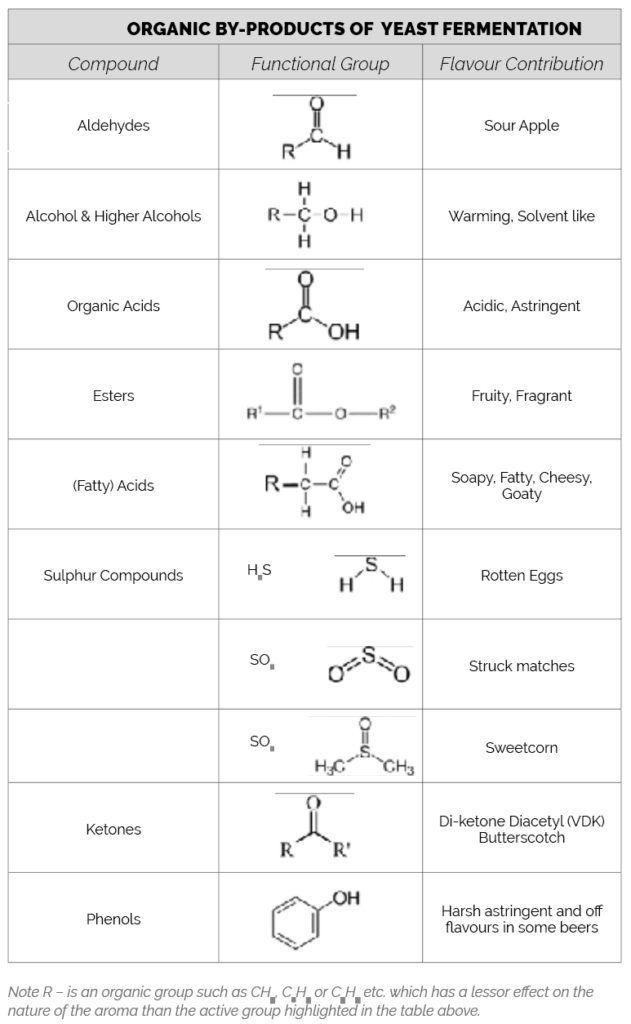
During fermentation yeast takes up nutrients from the wort and excretes metabolic by-products which give beer its characteristic aroma and taste. Flavour compounds can be grouped into families based on their active site which connect with our olfactory receptors giving the impressions of aroma as outlined below.
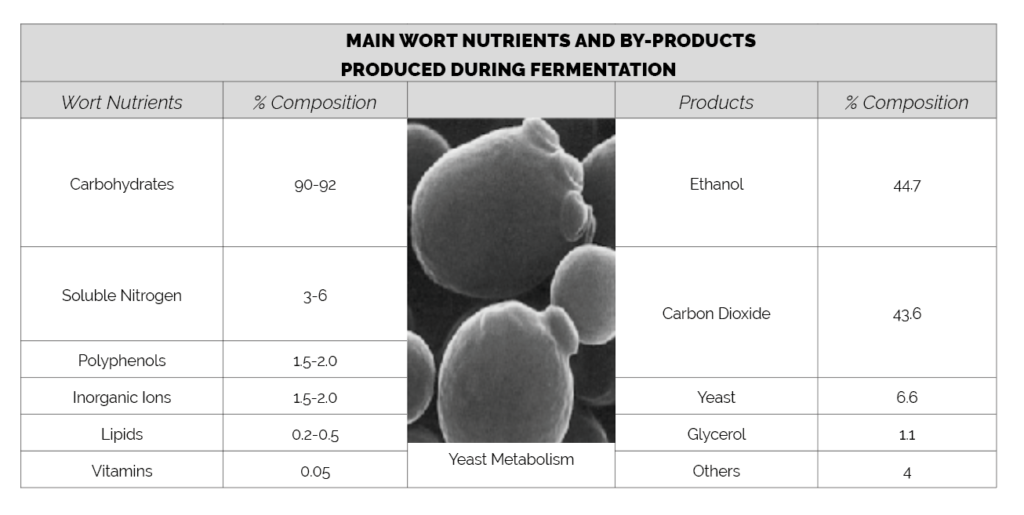
Yeast takes up wort nutrients to grow and produce more yeast release energy from carbohydrates as discussed in Article 4 (Carbohydrate Metabolism). Under anaerobic conditions the yeast produce approximately equal concentrations of ethanol and carbon dioxide.
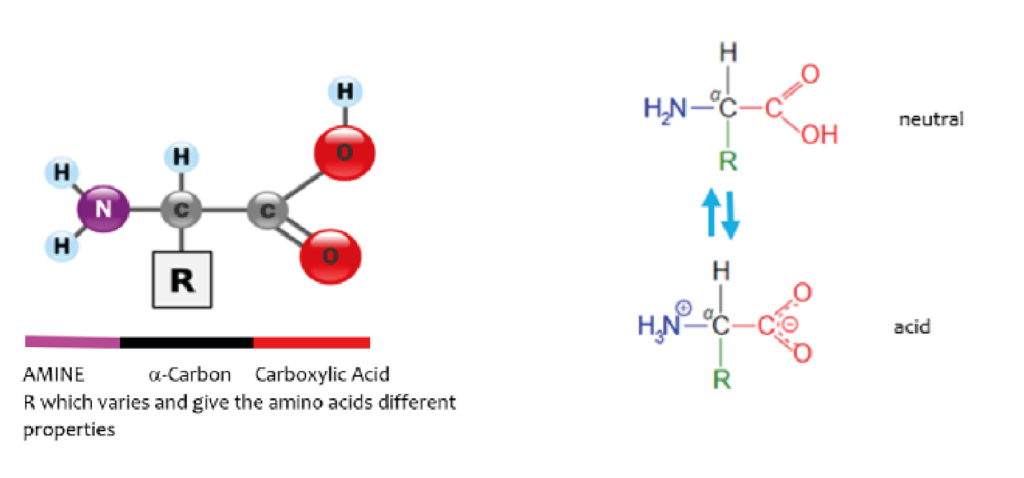
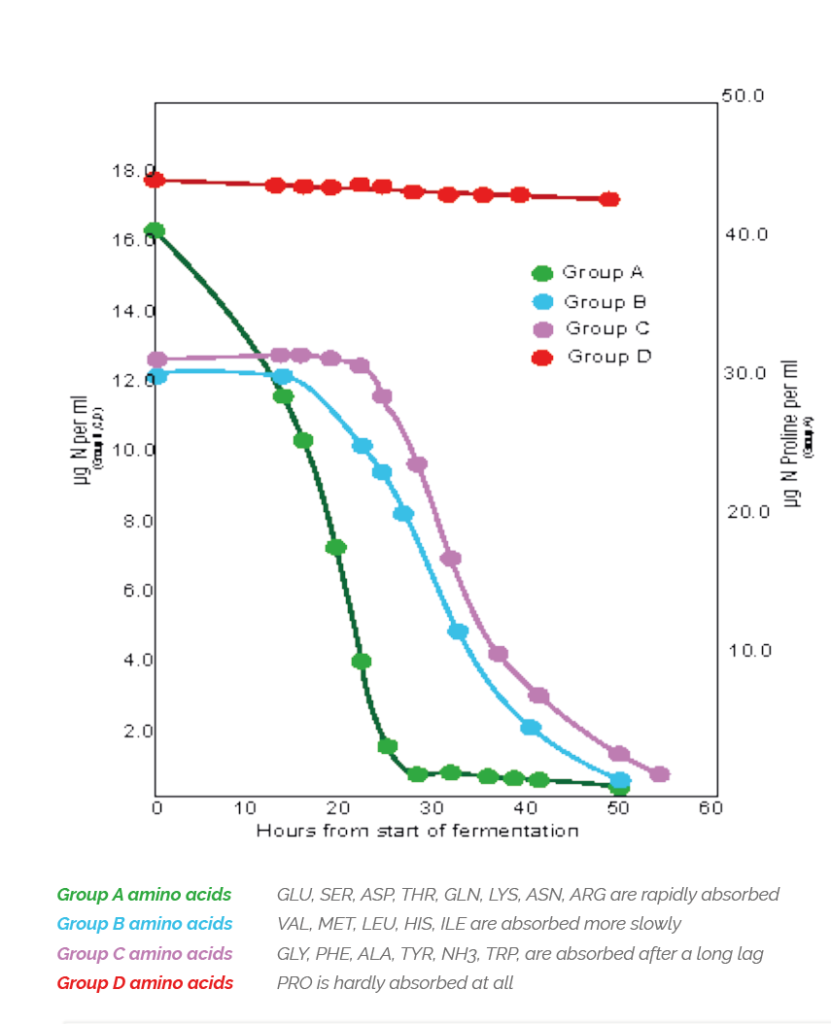
As well as energy, yeast requires and produces structural proteins and enzymes for growth. In brewer’s wort the amino acids are available from protein breakdown (expressed as FAN – free amino nitrogen) during malting and mashing and all, except for Proline, are absorbed by yeast.
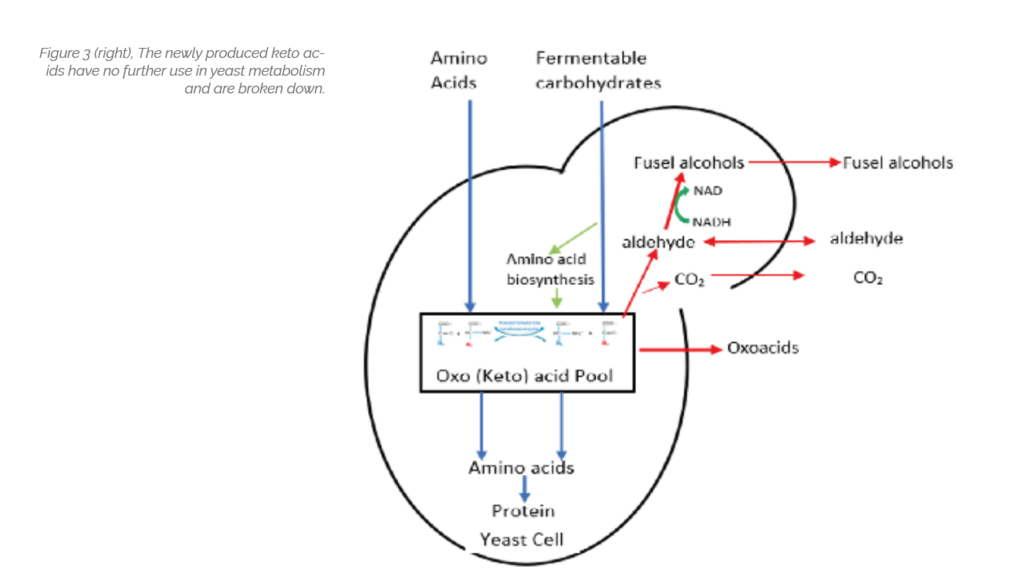
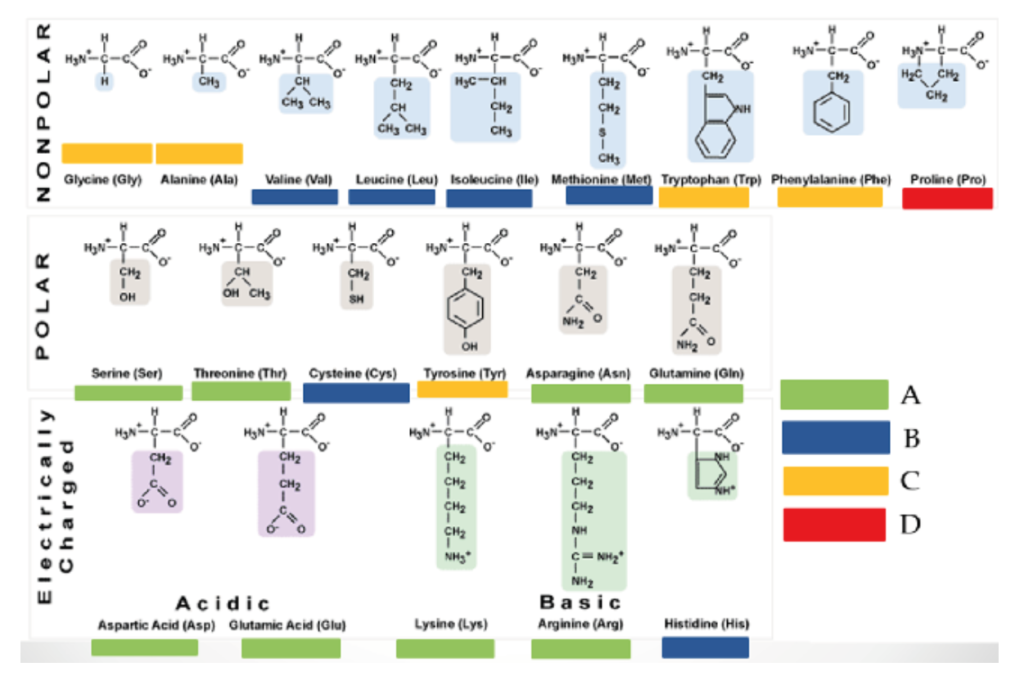
Like carbohydrates, yeast take up the available amino acids in a particular sequence depending on structure. Yeast requires the full range of amino acids throughout its growth stage to produce the different proteins required and while these could be synthesised from NH4 salts it takes more energy than converting an existing amino acid and replaces the structural group [R] with another group available from a keto acid in a process called transformation shown below.
The yeast has a large reservoir of Keto acids – the “keto acid pool” which it can access to produce the required amino acids for protein synthesis.
The newly produced keto acid has no further function in yeast metabolism and represent a threat to the cell in terms of ionic balance (Redox potential). The cell therefore eliminates it through decarboxylation (removing of CO2) to produce the equivalent aldehyde.
During catabolism yeast produces energy in the form of ATP which is used for growth. During anabolism new growth molecules are synthesised using energy from ATP which is recycled to ADP.
As part of carbohydrate metabolism, yeast also produces another high energy compound NADH (from NAD). Yeast produces an excess of NADH which must be recycled. During aerobic respiration this is achieved by oxidation with molecular oxygen. Under anaerobic fermentation yeast must reduce the NADH via other compounds.
A variety of chemical reactions are used to oxidise NADH to NAD usually by reducing double bonds shown below. All reactions, apart from the reduction fumarate to succinate, involve the reduction of carbonyl groups (C O) to alcohol or aldol groups (HC OH) with the oxidation of two NADH.
A significant flavour active family of compounds produced during fermentation are during the end stages of fermentation (and warm maturation) to alcohols as shown below.
In effective fermentations, aldehydes are rapidly reduced to alcohols which have a lower bland or slight fruit flavour aroma. Higher levels of aldehyde indicate a defective fermentation. These “higher” alcohols (with higher molecular weight than ethanol) are often described as “Fusel alcohols” and along with aldehyde are believed to be responsible for “hangovers”.
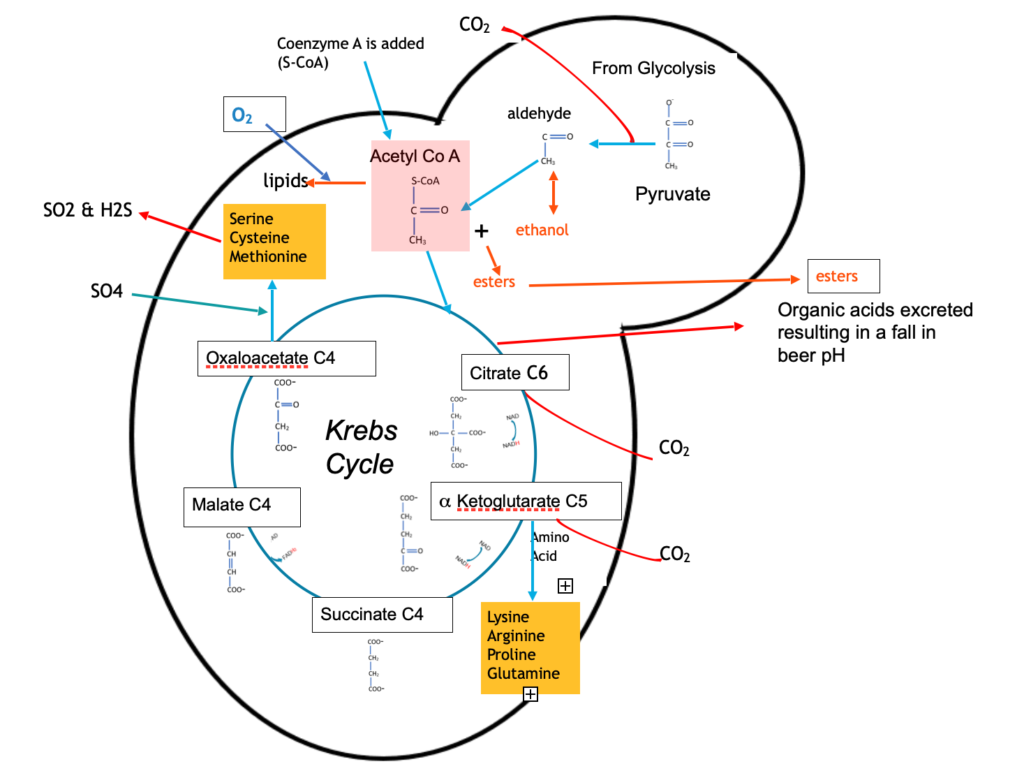
The Krebs cycle (also known as the Citric Acid Cycle) is an essential step in the breakdown of sugars to CO2 and water during aerobic respiration but has lesser function during anaerobic respiration (fermentation) where it is part of the metabolic pathway for the synthesis of essential amino acids and organic acids which are excreted to drop the pH from around 5.2 in wort to 4.0 in finished beer.
As well as providing a source of amino acids the Krebs cycle gives off CO2 and produces NADH which must be oxidised to NAD.
One of the most flavour active compounds in beer are Vicinal Diketones consisting of 2,3 butanedione (diacetyl) and the related compound 2,3 pentanedione. They are formed by decarboxylation of a keto acid to form flavour active compounds. Diacetyl and 2,3 pentanedione, these are then rapidly reduced by yeast to recycle additional NADH.
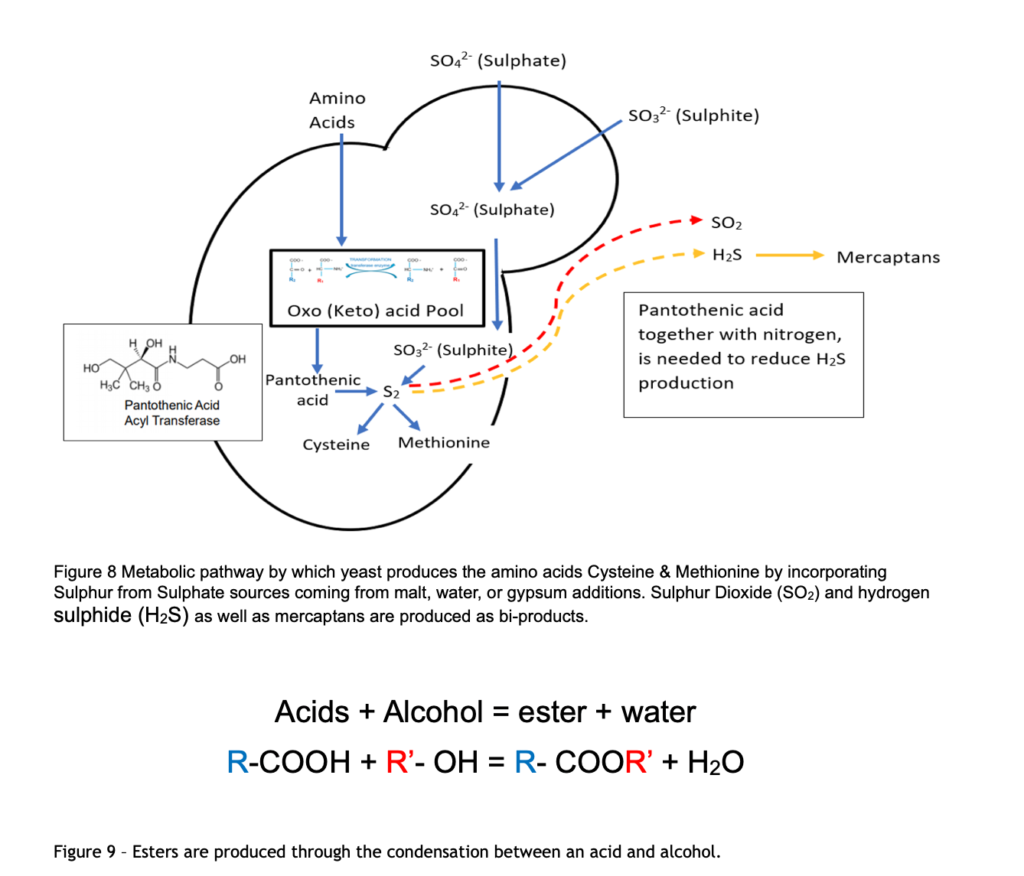
There are sulphur based amino acids, Methionine (Group B) and Cysteine (Group C) which are synthesised by transamination of other amino acids using sulphate salts derived from malt, water or added gypsum and as a bi product of this metabolism yeast produces sulphur dioxide (SO2), Hydrogens Sulphide (H2S).
Many of these bi-products persist in the finished beer, but some react to form other significant flavour active compounds.
Alcohols react with acids to form esters. Acetyl CoA is the most plentiful acid, and this reacts with the most abundant alcohol, ethanol, to produce two principal esters:
- Ethyl acetate which has a flavour threshold of 18 mg/l giving a fruit flavour often described as nail varnish remover.
- Iso-amyl acetate which has a flavour threshold of 1.8 ppm giving a fruit flavour often described as bananas.
These are also a range of minor esters produced by the condensation of Acetyl COA with higher (more complex) alcohols to give beer a range of pleasant fruity flavours. Many esters are present at levels below their individual flavour detection threshold but combine to give a fruity impression to beer.
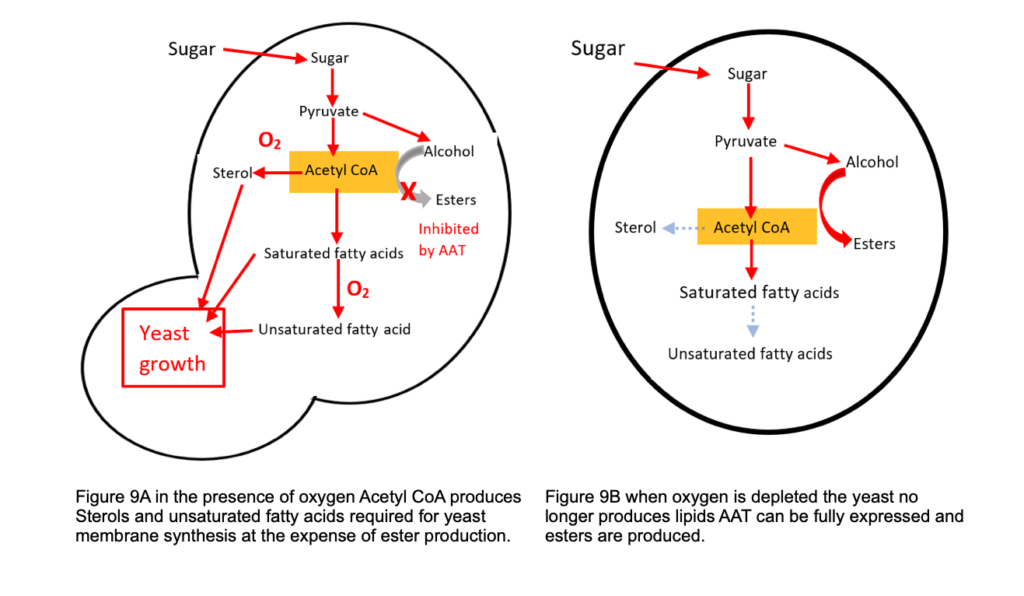
Acetyl CoA is highlighted in Figure 4 as an intermediate metabolite for many reactions. Although fermentation involves anaerobic respiration (in the absence of oxygen), air or oxygen is added at the very start of fermentation where it is used to produce yeast membrane material essential for growth.
As discussed in B2B2 “Yeast Structure” published in January 2021 yeast uses the oxygen to produce unsaturated fatty acids and sterols for membrane synthesis. This takes priority over ester synthesis which uses the enzyme AAT (alcohol acyl-CoA transferase). The production of this enzyme is inhibited in the presence of oxygen and unsaturated fatty acids reducing the expression of the ATF1 gene responsible for ester production.
The development of flavours during fermentation is delicately balanced and depends on yeast strain and fermentation conditions, for example the level of oxidation will affect the amount of esters along with fermentation temperature and pressures. The gives the Brewer ways of controlling beer flavours to be discussed in a later edition of this series.
As explained in a previous article, Brewer’s Yeast is highly adapted to grow in the wort provided and can secure its future by rapidly dropping the pH (through excretion of organic acids), produce toxic alcohol and make the fermentation into an anaerobic environment thus stifling many of its microbial competitors.
This makes fermenting beer a great place for yeast to bring up its children and during the growth phase it produces a large amount of bi-products (overflow metabolism), some contribute to the positive flavour of the beer, while others have a negative flavour contribution which are mainly reduced in a vigorous secondary fermentation (warm Maturation).
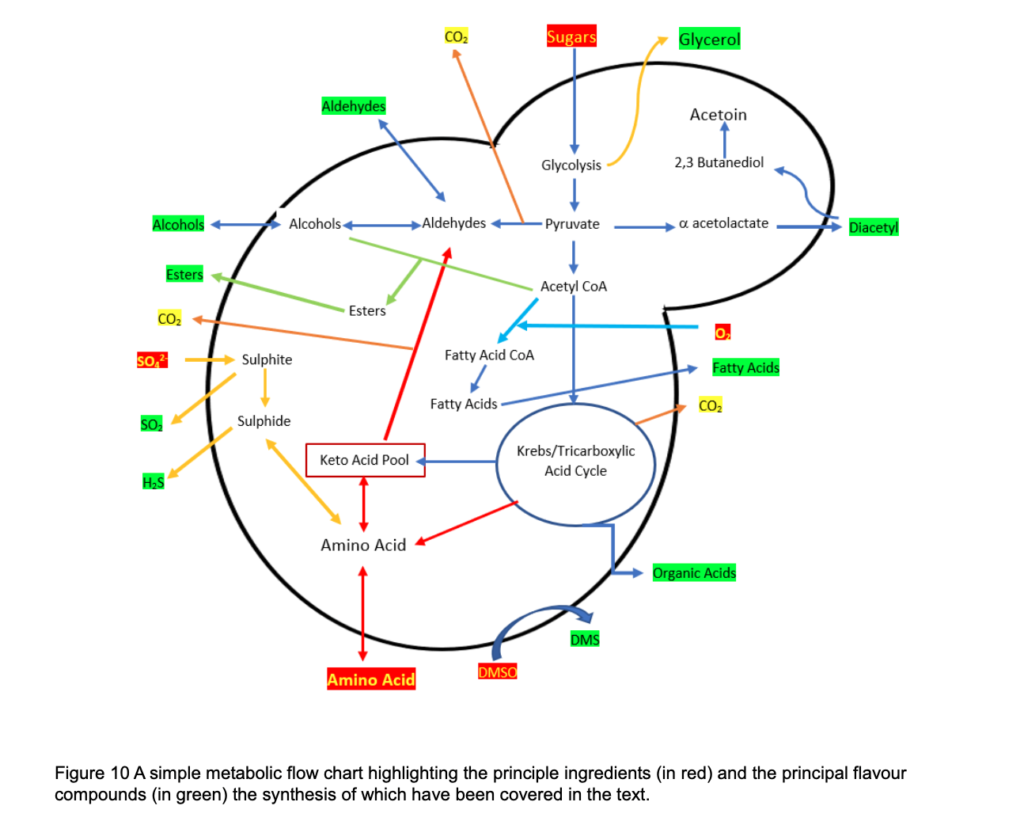
The principle flavour compounds, and metabolic pathways are summarised below.
Acknowledgement: I would like to thank Professor Graham Stewart Emeritus Professor Heriot Watt University and Dr Sylvie Van Zandycke from Lallemand for checking the metabolic details. Any errors are entirely down to the author.