You’ve heard of thiols, but are unsure what the fuss is about. Here is Alix Blease from Lallemand to explain all…
In chemical terms, a thiol is a molecule which contains a terminal -SH group. As a brewer you are probably already familiar with at least one thiol, 3-methyl-2-butene-1-thiol (3MBT), which is responsible for the ‘skunky’ off flavour in light-struck beers.
Many thiols have very strong odours which range from garlic, sweat and onions to tropical fruit, wine and citrus.
Because of their aromas they are often used as odorants. As Ross from Friends once taught us all, “that smell is added to natural gas so that you know if there is a leak” – well that added smell is the thiol ethanethiol.
You don’t want your beers to smell like onions, garlic and gas, but what about the nice smelling thiols, how do you get those into your tropical IPA?
Thiols can be present in two forms, free (aromatic) or bound precursor molecules (odourless). It’s those bound precursors which need to be released and converted into the desirable and trendy free aromatic thiols to give your beer more of a fruity punch.
Some of the common thiols which are considered as being positive in beers are 4-mercapto-4-methyl-pentan-2-one (4MMP or 4S4MP), 3-mercaptohexanol (3MH or 3SH) and 3-mercaptohexyl acetate (3MHA or 3SHA).
They contribute aromas of blackcurrant, tropical fruits, citrus, grapefruit and passionfruit.
So the more free aromatic thiols the better, right? Wrong, thiols have an incredibly low flavour threshold in the nanograms per litre region (which is why ethanethiol is added to gas because we can smell it at levels one hundred million times lower than levels of ethanol).
If you create too many free thiols then you can get vegetal, sweaty, rubbery and over-ripe fruit characteristics in your beer.
So how do you go about maximising thiols in your juicy NEIPA recipe? Free thiols and their precursors and are found in hops, malt and grapes, as well as in lower quantities in rice, sorghum and wheat. In hops the free thiols and precursors are referred to as the “sulphur fraction” and make up less than 1% of all of the hop essential oils.
Free thiols are present in a lot of hop varieties. 4MMP (the blackcurrant thiol) exists more commonly in its free form than it’s bound precursor in hops such as Citra, Mosaic, Simcoe and Sorachi Ace.
The bound precursor molecule of 3MH (the citrus and grapefruit thiol) is present in extremely high quantities in Cascade.
A study of Cascade found that the concentration of precursor thiols are higher in hops harvested earlier in the season.
Over the course of the harvest this concentration decreases and the concentration of free thiols increases proportionally. Malt only contains bound precursor molecules. The 3MH thiol precursor is found at the highest concentrations in malts with the lowest amount of kilning, or lowest EBC value.
Interestingly, freshly harvested barley is actually relatively low in thiol precursors and in most cases the process of malting actually increases the concentration of precursor molecules.
So you’ve designed a recipe with pale malts and hops high in bound thiols, how do you go about releasing these aromatics? Well, unsurprisingly for an article written by someone from Lallemand, the answer is in yeast! The ICR7 gene in Saccharomyces cerevisiae codes for an enzyme called beta-lyase.
Beta-lyase releases the thiols which are bound to cysteine (Cys). Unfortunately, >90% of the bound thiol precursors in hops and malted barley are bound to glutathione (Glu) and not Cys. Therefore, it’s not as easy as just “ferment your beer with yeast”…
So, what can you do to maximise these thiols?
– It has been observed that peptidase enzymes present in malt have the ability to convert some Glu-thiols into Cys-thiols. Therefore, consider a protein rest stage during the mash to allow the Glu-thiols in the pale malts to convert into Cys-thiols. You could also add hops high in Glu-thiols (Cascade and Simcoe) at this stage.
– Bound precursors can be converted into free thiols during the boil, however they are incredibly likely to be lost as they are extremely volatile. Focus on maximising the precursors available on hot-side.
– Whirlpool hop additions also risk driving off volatile free thiols, but adding hops at this point high in Cys-thiols increases precursors for the entire duration of the fermentation.
– Dry hopping earlier in fermentation with hops high in Cys-thiols (Citra, Simcoe and Mosaic) increases the opportunity for the conversion to free thiols by the beta-lyase in yeast.
– Dry hopping at the end of fermentation, with hops high in free thiols (Citra, Sabro and Mosaic) will increase thiol levels immediately and ensures they won’t be driven off by CO₂ during fermentation.
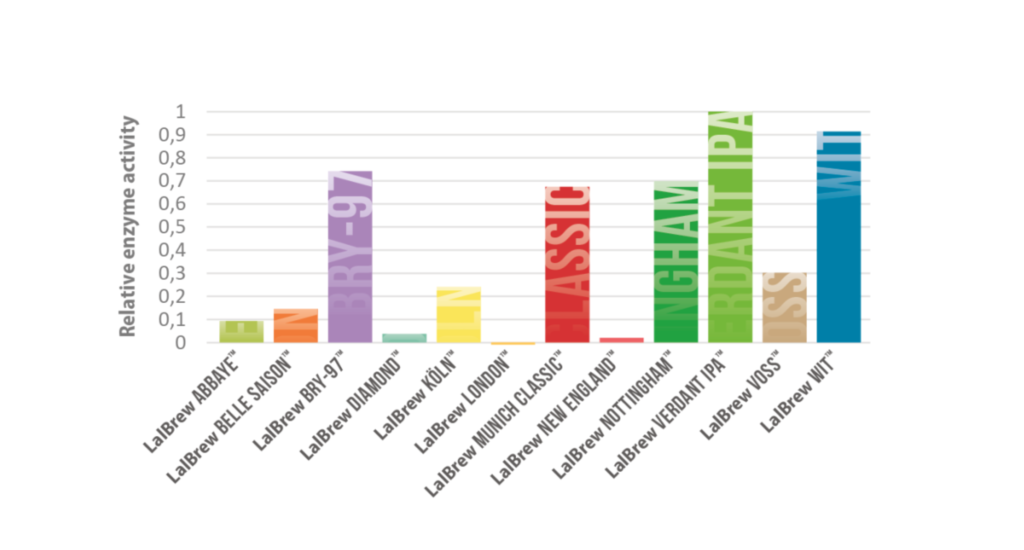
– Beta-lyase enzyme activity varies between yeast strains. Select a yeast strain with high enzyme activity to maximise the release of bound thiols.