This brief review will illustrate how brewers may obtain such alcohol and beer extract values and then, along with optional beer protein estimation, shows how to approximate both total beer carbohydrate, and calorie information with a reasonable degree of accuracy. The approaches here allow the brewer to obtain such information using basic instruments and robust algorithms. Though the data as obtained are to be considered as unofficial results for internal use only. For legal reporting purposes, the brewer must have access to expensive and officially accepted instruments for their measurements or make use of a qualified and established third party analytical facility.
If seeking an analytical testing facility for beer alcohol, extract and nutritional work, the brewer should be sure the facility fully understands how to test the beer and report the parameters. Data must be obtained using officially approved instrumentation and methods as quickly, efficiently, and accurately as possible while all being done at a reasonable cost. The brewer in the US might also ask if the lab is TTB certified or otherwise recognized as providing reliable data. For European brewers and Canadian brewers there are agencies that they can consult and laboratories available to test their products. With that said we now look at some basic evaluations and calculations to better understand key beer parameters no matter if products are tested in house or elsewhere. As this article is directed towards a British and Canadian brewing perspective, calories will be expressed in both US Calorie and International kJoule units.
Alcohol and extract determinations
Careful measurement of the original extract (OE) of the wort and terminal gravity of the beer can lead to the calculation of attenuation (fermentation efficiencies), and to determination of residual extracts (real and apparent) and alcohol content. A review on how to determine alcohol content and extract values has appeared recently (Spedding, 2016); the reference shows the use of proper tools, methods, and robust algorithms needed to obtain accurate data. Currently user error, both through incorrect instrument use and method performance and the use of simple formulas (often inaccurate “rule of thumb” homebrewer’s equations), is the main cause for many calculation discrepancies.
The understanding of alcohol and extract determination is underpinned by a theory pertaining to fermentation and alcohol production, chemical mass balance relationships of fermentation and, Carl Balling’s original theoretical work in brewing science. This topic has been covered elsewhere (Spedding, 2013, 2016 and references contained therein). To keep this present article as brief as possible only the details of the calculations needed by the brewer to obtain key data are presented.
For brewers to be able to determine both the alcohol by weight and by volume for beer the following values must be known or obtained; the present or apparent gravity of the beer, the real extract of the beer and the original extract of the wort. To help solve for these various terms an arithmetical relationship exists between them as attributed to Carl Balling. The relationship which is known as Ballings’s formula is:
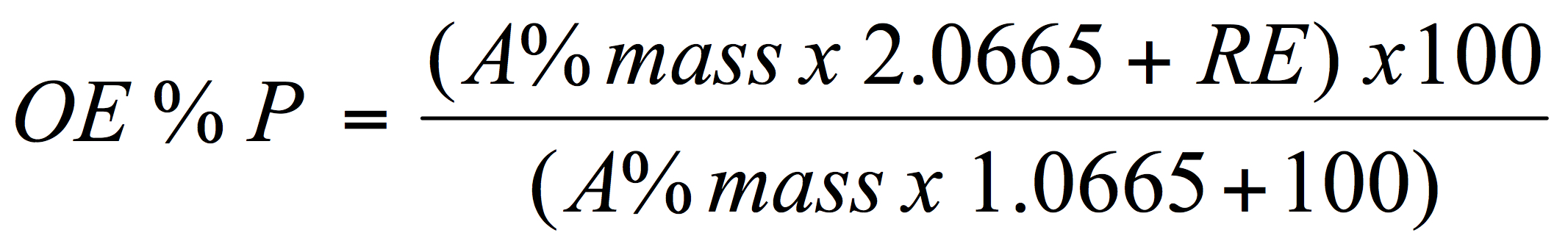
Where: OE is original extract (Plato; g/100g or mass/mass), A% mass is alcohol by weight, RE is the real extract and the numbers in the numerator and denominator are as described below.
The above formula (equation 1) is based on an understanding of the mass balance relationship in brewing – simply the chemical relationship dealing with the conversion of fermentable sugars to alcohol, carbon dioxide and yeast biomass. Theoretically, 1 gram of fermentable sugar will yield 0.51 gram of ethanol and 0.49 gram of carbon dioxide. In fact, some sugar is needed for cell growth and so, more realistically, the ethanol yield is more likely 0.46 gram, and carbon dioxide 0.44 gram from 1 g sugar. Or more simply put: 2.0665 g sugar yields 1 g ethanol, 0.9565 g CO2 and 0.11 g yeast and – for the equations below: 0.9565g and 0.11g sum to 1.0665g which is the extract not converted to alcohol.
An application of Balling’s formula and a minimum number of analytical measurements allows the brewer, without the full range of sophisticated instruments to obtain some quite accurate values for alcohol and extract using a series of approximate beer calculations.
In simplifying the discussion, it is known, from above that a quite accurate estimate of alcohol content can be derived by subtracting, from the original extract (OE), the final or present extract gravity (PG) as “real extract” (RE – not the apparent extract, AE), assuming no process dilution, and by applying conversion factors which infer the alcohol content from the drop in the wort gravity occurring during fermentation. Using an equation, deriving from Balling’s formula noted above, the alcohol by weight can be calculated:
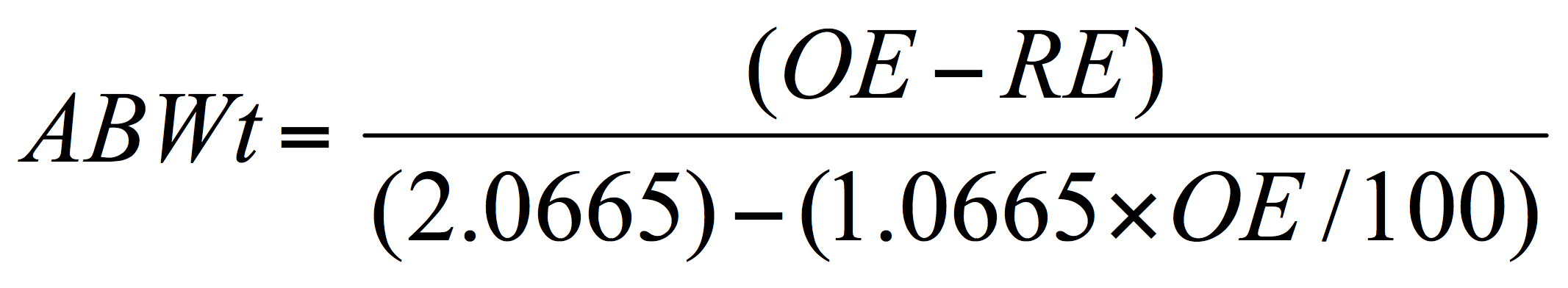
Where ABWt is the percentage alcohol by weight (as w/w, grams of alcohol per 100 grams of beer), OE, original extract and RE the real or present extract in degrees Plato. It is assumed that the brewer is familiar with or will familiarize themselves with all these brewing terms.
The original extract of wort is the amount of material extracted from the mash and is measured in grams of extract per 100 grams of wort. The brewer will determine the original extract by measuring the specific gravity of the wort and will relate this to the established sucrose (= extract) tables (in grams per 100 grams) to report the original extract content in degrees Plato. Or more simply they will determine the OE using a Plato hydrometer.
For the brewer limited to the use of a hydrometer, real extract (RE) can be approximated using the OE (of the original wort) and AE (apparent extract of the final and degassed beer) and by using another empirical equation recently revised but based on Balling’s work:
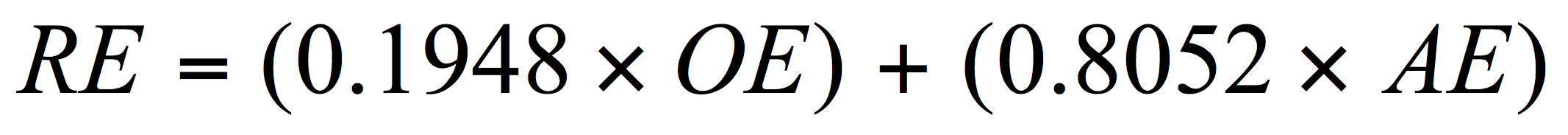
(AE = apparent extract – that extract value determined when alcohol is present. This is the value for gravity obtained by the brewer on the final attenuated beer.)
The real extract (RE) assessment here will always be an estimate for reasons discussed elsewhere (Spedding, 2016). Using equation 3, the brewer will find satisfactory answers to RE values compared to those obtained from official instrumentation over the typical ranges of OE, real degree of fermentation (RDF), and alcohol for most beers (see Spedding, 2013 and 2016 for a fuller discussion on limitations to the above approaches). Errors in evaluations between official and less accurate methods are typically quite small but are not considered further here. Operators should be aware of accuracy and precision for all methods and instruments used.
Getting back to the use of Plato values – by substitution in the above formula (Eq. 3), it is possible to solve for the alcohol by weight without directly inputting a calculated or determined RE value:
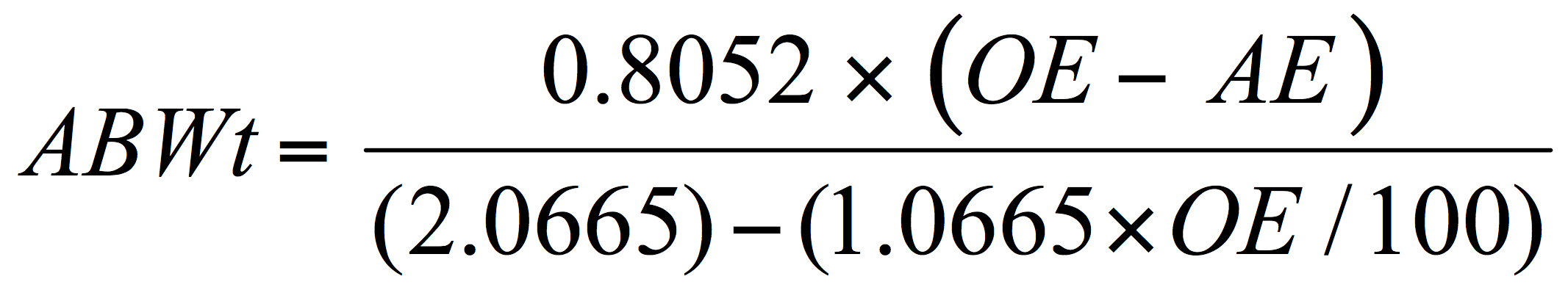
And then, if the SG value for the beer has also been determined the alcohol by volume can be deduced with a correction to account for the specific gravity of the beer:
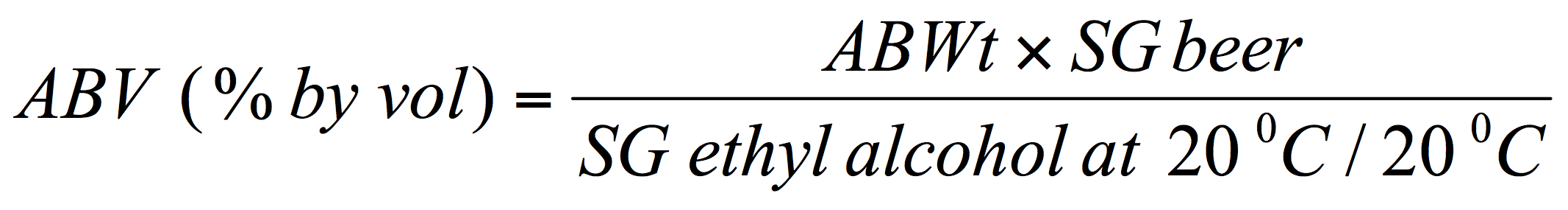
[Where, SG is the specific gravity, e.g., for the beer or pure ethanol respectively.]
This simplifies to:
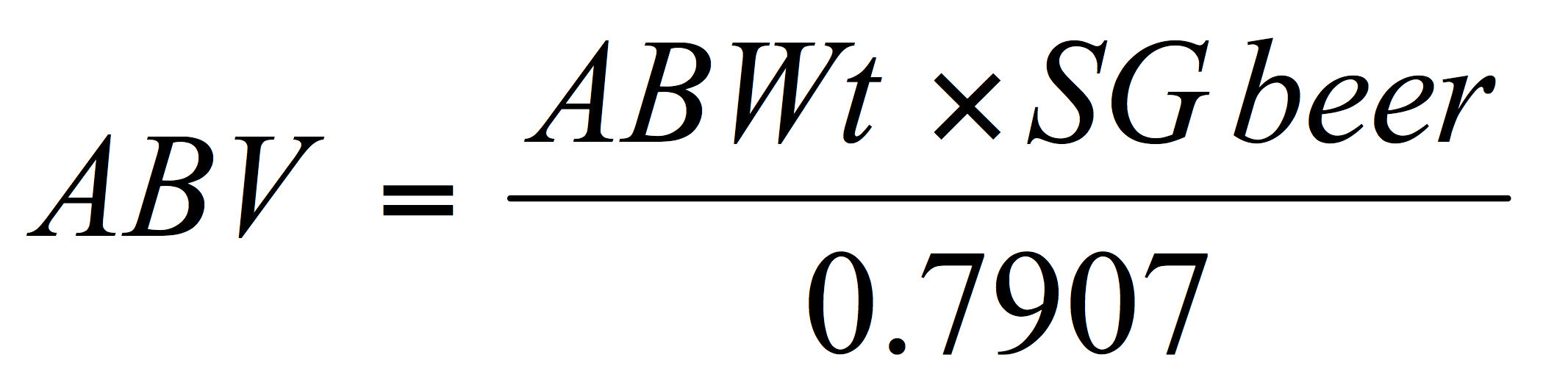
Variant equations are available but alcohol should be reported both % by weight (wt.) and by volume (vol.) to two decimal places. For reporting purposes brewers in the US are allowed a tolerance of +/- 0.3% alcohol by volume (see American Society of Brewing Chemists, 2017).
Armed with the set of principles, equations and tables defined or referenced above – if the brewer can obtain either reasonably accurate Plato extract values or SG values for the original extract (gravity) of their wort and the terminal (apparent) extract gravity of the resulting beer – as well as real extract (measured or calculated), via use of hydrometers, refractometers or simple digital density meters, they can get quite close to true alcohol results for beers with low to reasonably high alcoholic strengths – typically 2.5-12% ABV and real extract values of around 2.5-8 degrees Plato (see caveats in Spedding, 2016). Furthermore, with the real extract value for the beer in hand several other useful equations can be applied as described below.
Basic Nutritional Calculations
With alcohol by weight, real extract and total beer protein determined it is possible to obtain a good estimate of total carbohydrate. Protein values will need to be obtained from a third-party lab and this complex topic has been discussed elsewhere (Weygandt et al, 2017). For a more accurate carbohydrate determination a value known as ash (which is the inorganic mineral content in the beer expressed as % by weight per 100 mL – w/v) will also be needed; ash determination has also been discussed by Weygandt et al, (2017). Established brewers know how to calculate total calories in their beers starting with just alcohol and real extract information; many more craft brewers should now learn how to do so. In Europe researchers also showed that, for the most part, a set of routine calculations solve the overall calorie values for many of the beers they tested (Olšovská et al, 2015).
For simple nutritional purposes, we can consider that traditional beers contain mainly alcohol, carbohydrates, and protein. For unofficial in-house estimation, the ash determinations may be omitted from certain nutritional calculations (see below). The more detailed examination of the topic presented by Weygandt et al, (2017) should again be consulted for more official nutritional reporting claims.
Carbohydrate calculations. Once the protein, ash and extract values for the beer are known the total carbohydrate value is obtained by the formula: Carbohydrates g/100g beer = Real Extract – protein – ash.
An example will serve to illustrate the total carbohydrate content in a robust porter. Note: a typical serving size as in the US – 12 US fl. oz (355 mL) is used in the examples below. It should be easy to see how to adjust for a different serving size.
Porter
6.40°P = real extract, (°P = degrees Plato, g/100g or % by weight); 0.70 = protein % by weight; and 0.22 = ash % by weight; 1.01534 = Specific gravity (SG) of beer; 355 = 12 fl. oz. in mL.
Carbohydrate g/100g beer = 6.40 – 0.70 – 0.22 = 5.48 g
Carbohydrate/12 fl. oz. beer = 5.48 x (355 x 1.01534/100) = 19.75 g
(See Beer-6 in the ASBC Methods of Analysis – American Society of Brewing Chemists, 2017 for further details. The European Brewery Convention also supplies a special methods manual that covers the same details.) A final note here covers the fact that in the absence of protein and ash data the real extract value can be taken as an upper limit on the carbohydrate content of the beer – a rough upper estimate! Though use this value as a crude estimate only if anyone requests the carbohydrate load of a beer style, and note also that some of that carbohydrate is non-calorie bearing (again see Weygandt et al, 2017).
Calories, energy and definitions
In the US, the term calorie refers to a kilocalorie. One kilocalorie (kcal) is the same as one Calorie (upper case C). A kilocalorie is the amount of heat energy required to raise the temperature of one kilogram of water by one degree Celsius. Nutritional calculations are largely based on the so-called Atwater factors, which express the caloric content per nutrient source (fat, protein, carbohydrate, alcohol) in terms of calories per gram. For protein and carbohydrate this is 4 kcal/gram, fat 9.0 kcal/gram, alcohol 6.9 kcal/gram (7.0 in Atwood tables though brewers’ use 6.9). The Atwater factors are discussed elsewhere (University of Minnesota, 2017). The international or SI unit for measuring food energy is the joule with nutritionally relevant amounts of energy expressed in kilojoules (kJ, 1000J). 1 US calorie is 4.18(65) joules or 1kcal (Calorie) is 4.18kJ (usually rounded to 4.2). The related Atwater factors in kilojoules are: protein, 16.8 kJ/gram (rounded to 17), fat, 37.8 kJ/gram (not relevant to us here), carbohydrate 16.8 kJ/gram (rounded to 17) and alcohol at 29 kJ/gram (Hughes and Baxter, 2001).
As total beer calorie values are sometimes important to consumers and for reporting to official regulatory bodies we provide the total calorie calculations that will be of most use to the brewer. An example for total calorie determinations in beer as based on calculations described in ASBC Method Beer 33 again includes the robust porter beer data as noted above for the carbohydrate determination.
Porter
Alcohol by weight; 5.54%, real extract; 6.40 Plato (% by weight), ash; 0.22 (% by weight) and SG of the beer 1.01534.
Calories per 100 grams of beer: = 6.9 * (5.54) + 4 * (6.40 – 0.22) = 62.95.
[The real extract minus the ash is considered as protein and carbohydrate and it will be recalled from above that there are 4 kcal of energy per gram for both protein and carbohydrate.]
Calories per 12 fl. oz. (355 mL beer). = 62.95 * (355 * 1.01534/100) = 226.9. [226.9 Calories or kcal.]
As already mentioned above the brewer could omit the ash value and still obtain a suitably accurate value of the calories if the alcohol by weight and real extract values have themselves been obtained accurately. From the data above the astute reader should be able to back-calculate the alcohol by volume for this porter beer (hint it is slightly above 7.0%)
In the UK, the calorific (energy) value is calculated in a related way to the above using a formula based on Food Labeling Regulations:
Energy value (kJ/100 gram) = (alcohol * 29) + (carbohydrate * 17) + (protein * 17)
Simplifying and incorporating the ash value:
Energy value (kJ/100 grams of beer) = 29 * (5.54) + 17 * (6.40 – 0.22) = 265.7
For the example: kJ/12 fl. oz. (355 mL beer). = 265.7 * (355 * 1.01534/100) = 957.7 [957.7 kJ]
If using the US approach, simply multiply the kcal number by the rounded conversion factor number 4.2 to obtain the kJ value. From the above example, the values would then compute out to be 264.4 kJ/100 grams of beer or 953.0 kJ/12 fl. oz serving of the beer.
Depending on exactly how calculated (using either the accurate or rounded values), these values will vary a little but should provide an adequate means to obtaining data that will be within most tolerated reporting ranges.
In the US, the statement of caloric content on labels for malt beverages will be considered acceptable if the caloric content, as determined by approved TTB analysis, is within the tolerance +5 and -10 calories of the labeled caloric content (https://www.ttb.gov/rulings/80-3.htm). For example, a label showing 96 calories will be acceptable if the analysis of the product shows a caloric content between 86 and 101 calories. European and Canadian regulations may be slightly different and the brewer should always be aware of local and changing regulations and the tolerances for reporting caloric content in kJ terms.
Conclusion
Any brewer routinely getting alcohol and extract data and (optionally) ash and protein values can get quite good estimates of their beer’s carbohydrate content and note the caloric impact per serving size. Furthermore, they will be better informed on their own brew-house efficiencies, as well as providing consumers of their beers with nutritional information to help them make better choices in their drinking of fine craft and homebrewed beers.
The brewer should also check out the Scandinavian Beer calculator to perform a range of calculations including calories in kJoule units (http://www.beercalc.com/). However, note how the calculator is not using an ash term for calorie determination. It simply takes the real extract as if all protein and carbohydrate as best we can tell. The values should all be good enough for most purposes. The reader should practice with this robust and freely available on-line tool.
For those brewers with a wider distribution of their products, and whose beers might come under the recent US and European nutritional mandate guidelines, more extensive and official testing will be required (consult local regulatory authorities, the author, BDAS, LLC, Olšovská et al, 2015 and Weygandt et al, 2017 for current details). Perhaps most significantly though, is that those brewers not paying more attention to quality considerations may not survive in an ever more crowded and competitive marketplace. Product consistency is a hot topic today and this review on alcohol measurements and nutritional basics should, hopefully, provide a platform to help the brewer launch a quality control program to ensure delivery of the most consistent highest quality beer time after time.
REFERENCES
American Society of Brewing Chemists (2017). Methods of Analysis -14th Edition. American Society of Brewing Chemists. Beer 6: Calculated Values, Beer 11: Protein and Beer 33: Caloric Content (Calculated). http://methods.asbcnet.org/default.aspx [Access restricted to ASBC members. Last accessed, July 2017.]
Hughes, P.S. and Baxter, E. D. (2001). Beer: Quality, Safety and Nutritional Aspects. Royal Society of Chemists.
Olšovská, J., Štěrba, K., Pavlovič, M. and Čejka, P. (2015). Determination of the Energy Value of Beer. J. Am. Soc. Brew. Chem. 73(2); 165-169.
Spedding, G. (2013). Empirically measuring and calculating alcohol and extract content in wort and beer with a reasonable degree of accuracy and confidence – Using a series of inter-related and conversion equations, algorithms, tables and an on-line calculator. Brewers Digest. Sept-Oct; 39-50.
Spedding, G. (2016). Alcohol and its measurement. In: Brewing Materials and Processes: A Practical Approach to Beer Excellence. (Charles W. Bamforth, Ed.). Elsevier/Academic Press. pp. 123-149.
University of Minnesota (2017). Atwater factors. http://www.ncc.umn.edu/products/nutrients- nutrient-ratios-and-other-food-components/primary-energy-sources/ [Last accessed, July 2017.]
Weygandt, A., Linske, M., Gennette, P. and Spedding, G. (2017). Beer Analysis and Testing. The New Brewer. Vol 34(4): July/August 2017; 70-72, 74, 76, 78, 80, 82 and 84.
Notice
The content has been produced by Gary Spedding, a brewing and distilling analytical chemist and biochemist with a special interest in the origins and development of beverage flavour and in the sensory evaluation of beer and spirits. His company, Brewing and Distilling Analytical Services (BDAS, LLC), a triple TTB certified laboratory, was founded in 2002 as an unbiased and dedicated facility for testing alcohol containing beverages and beverage raw materials. The content in this review is for informational purposes only. The article has been prepared with a view to instructing the brewer using hydrometers, refractometers and or simple density meters to obtain “reasonably approximate” values for routine evaluations; data to be used in-house only.
It assumes the data are collected both correctly and as accurately as possible using such instruments. Those using both Plato and specific gravity hydrometers will be set for most tests – those using one type or the other should be aware that they can interconvert between the two units via the use of tables or algorithms which are detailed in some of the cited references. For official work, density meters, such as those from Rudolph Research Analytical or Anton Paar, are recommended if obtaining the OE of the beer wort and the final density and specific gravity information for use with the above noted alcohol and extract equations. Density and specific gravity interconversions should also be understood by the brewer and this may be discussed with the author or instrument manufacturers. Such information is also to be found in the cited references.









